|
Welcome to a VIA Online Learning Tutorial: This site has been developed to provide learning materials to assist you to make complete and comprehensive radiographic examinations of animals.
Select Chapter:
1. BARIUM MEAL
2. ADDITIONAL GIT STUDIES [print this page]
3. EXCRETORY UROGRAPHY
4. LOWER URINARY TRACT
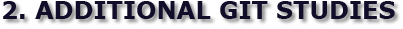
The Barium Enema
To properly evaluate the large bowel radiographically a retrograde contrast barium enema is the study of choice. For positive contrast we use liquid barium sulphate and for negative contrast we use room air.
The large bowel must be completely evacuated prior to any contrast examination. In order to ensure adequate bowel preparation solid foods should have been withheld for two days prior to the examination. On the day prior to the examination a faecal softener and laxative should be administered. Immediately prior to commencing the contrast study, survey radiographs of the abdomen should be made and if there is any residual faecal material visible in the large bowel it should be removed using the warm water enema.
The patient should be fully anaesthetised. A 'foley' catheter is inserted in the rectum and its balloon inflated. A 'purse-string' suture may be required to ensure that the inflated balloon of the catheter is retained within the rectum. Attach an enema bag containing barium sulphate to the foley catheter. The concentration of barium is 80-100% W/W. The volume used is subjective, being the volume required to distend the large bowel. In general, you should load approximately 20mL of liquid barium per kg. BW into the enema bag. Barium is slowly instilled into the large bowel and when approximately one half of the estimated volume has been instilled, a lateral radiograph is made.
If the large bowel is inadequately filled then add between a quarter and a half of the remaining liquid and take more radiographs. The positive study is complete when radiographs identify a fully distended large bowel and caecum. After you have taken your positive contrast set of radiographs, elevate the cranial end of the dog's body and drain as much liquid barium as possible into the enema bag. After this, inflate the large bowel through the foley catheter using room air. The result will be a double contrast barium enema. The mucosal surface of the large bowel should be coated with barium, but the lumen will be air-filled. This double contrast examination allows fine detail of bowel wall irregularities to be identified.
A second, much simpler and less messy procedure, can be performed using room air only. This quick and easy method can usually be conducted on a conscious animal which has been lightly sedated. With the patient in lateral recumbency, take a 100mL syringe with a catheter tip and fill it with room air. Place the catheter tip through the dog's anus and press the flat end of the syringe firmly against the anal ring. Inject the air and expose the lateral radiograph immediately the air has been injected. This will produce a negative contrast study of the rectum and terminal descending colon. It is useful to evaluate the terminal end of the large bowel for evidence of stricture formation, diverticuli and to differentiate gas distended small intestinal loops from the colon and caecum.
The full barium enema study is used to evaluate conditions of the upper large bowel and the ileo-caeco-colic junction. It is particularly useful for identifying ileo-colic intussusceptions and caecal inversion. It may also be used to evaluate evidence of neoplastic encroachment of the wall of the large bowel. Because most strictures and diverticuli are present in the lower colon or the rectum, the quicker negative contrast study may be quite adequate for their evaluation.
 Fig 1 Fig 1 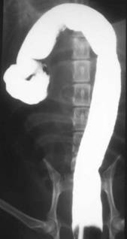 Fig 2 Fig 2
Positive contrast barium enema study
Negative contrast gastrography
Addition of air to the gastric lumen provides a negative contrast study of the gastric mucosa and enables detail gastric wall pathology to be visualised.
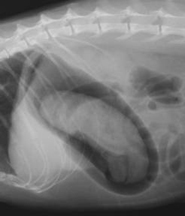 Fig 3 Fig 3  Fig 4 Fig 4
A gastric foreign body [a hairball] is defined using a negative contrast gastrogram
Another variation is a double contrast gastric study, which is achieved after a small volume of liquid barium has been administered, the animal is then rotated to ensure the barium coats the gastric mucosa. This is followed by air inflation of the stomach to achieve a double contrast effect.
Liquid iodine positive contrast studies:
There are occasions where it is preferable to use an iodinated compound rather than barium for upper gastrointestinal contrast studies. The main reasons cited to justify the use of iodinated compounds is to avoid complications that may follow barium contamination of pleural or peritoneal membranes. Some of these aqueous iodine solutions are hypertonic, and are not recommended for use in dehydrated patients. If a hypertonic solution is aspirated it may cause pulmonary oedema.

Fig 5: An aqueous iodine based contrast medium
Formulated specifically for gastro-intestinal studies.

Fig 6: Aqueous iodine products intended for intravenous
administration can be used as a substitute for specifically
formulated products.
Barium impregnated Poly Spheres [BIPS]
BIPS were created to study gastrointestinal motility. They can also be used to identify areas of intestinal stricture of obstruction. The impregnated spheres are packaged in gelatin capsules, each capsule containing a number of spheres. Usually there will be a mixture of small and large spheres, which is helpful when determining whether there is a partial or complete intestinal obstruction. The spheres are intended to be administered with food, so that their movement through the gastrointestinal tract mimics the movement of ingesta. This is more important for studies of gastrointestinal motility than it is for evaluation of suspected obstructions. The time taken for BIPS to accumulate in the large intestine and the pattern of distribution of BIPS through the gastrointestinal tract provides useful information for the observer. Each packet of BIPS contains a helpful booklet that describes distribution patterns of BIPS and how to interpret radiographic images containing BIPS.
 Fig 7 Fig 7 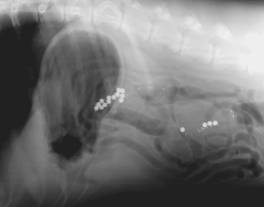 Fig 8 Fig 8
A BIPS package. Fig 2-13: Abdomen of a dog. This radiograph was taken
12 hours following administration of BIPS.
<< Previous chapter "Barium Meal" Next chapter "Excretory Urography" >>
|



